Convalescent plasma appears safe for treating COVID-19
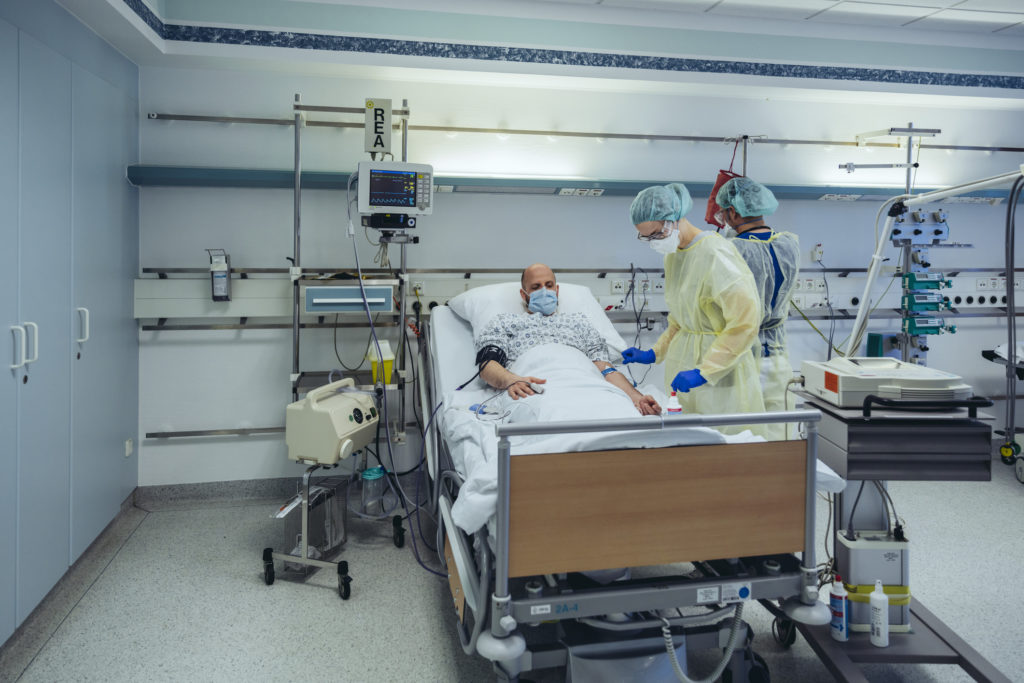
Transfusions of plasma from persons who've recovered from COVID-19, the illness that SARS-CoV-2 causes, seem to be safe for severely ill patients and could speed their recovery, according to an initial study.
For more than a century, doctors have used convalescent plasma (an element of blood) from people who survived life threatening infections to treat others.
The theory is that antibodies against a bacterium or virus stay in the blood of someone who has recently recovered from the infection.
Doctors used convalescent plasma as a treatment during the 1918 Spanish flu pandemic and had varying examples of success with it during the 2003 SARS outbreak, this year's 2009 swine flu (H1N1) outbreak, and the 2014 Ebola outbreak in Africa. In 2015, scientists established this treatment as the protocol for Middle East respiratory syndrome (MERS).
Early in the COVID-19 outbreak, on January 20, 2020, doctors in China started out treating severely ill patients with convalescent plasma. They reported encouraging results from five cases in the journal JAMA.
On March 28, 2020, Houston Methodist Hospital in Texas became the first academic infirmary in the United States to take care of critically ill COVID-19 patients with convalescent plasma.
“While physician scientists all over the world scrambled to check new drugs and treatments against the COVID-19 virus, convalescent serum therapy emerged as potentially one of the most promising strategies,” says Dr. James M. Musser, chair of the Department of Pathology and Genomic Medicine at Houston Methodist.
Study confirms safety
Between March 28 and April 14, Dr. Musser and his colleagues enrolled 25 people with severe or life threatening COVID-19 right into a preliminary study to research the safety of the treatment.
Nine of the participants (36%) showed a noticable difference in their condition after seven days, and 19 (76%) had improved or been discharged after 2 weeks.
There were no adverse events that the researchers could attribute to the treatment.
They report their findings in The American Journal of Pathology.
Among the concerns that persons have about the safety of convalescent plasma is that it could contain infectious agents, like the pathogen that it ought to be treating.
The authors report that the donors within their study had fully recovered and been asymptomatic for at least 2 weeks.
Commensurate with standard practices for donating blood, the researchers also screened the plasma for a variety of other pathogens, including hepatitis B and C, HIV, Chagas disease, West Nile virus, Zika virus, and syphilis.
They say that a lot more than 150 people who had recovered from COVID-19 donated their plasma to greatly help treat others, and many continue to do so.
“We are deeply indebted to your many generous volunteer plasma donors for his or her time, their gift, and their solidarity.”
- Study authors
Questions of clinical improvement
The researchers remember that the rate of clinical improvement among the persons who received the plasma was on an identical scale to the improvement that scientists reported in studies of the antiviral drug remdesivir.
However, they emphasize that the primary goal of their research was to measure the safety of the procedure instead of its efficacy.
The study was small, there is no control group, and the participants received other experimental treatments. For instance, 68% of them received the antiviral ribavirin, plus they all took the antimalarial drug hydroxychloroquine.
Notably, the authors report that there was no clear correlation between your amount of COVID-19 antibodies in infusions of donated plasma and the results for many who received them.
The study might have been too small showing a significant association of this kind, referred to as a “dose response.”
Houston Methodist is considering a more substantial, randomized manipulated trial of the therapy.
Concerns about timing
Other, multicenter studies of convalescent plasma are currently underway.
IN-MAY, a collaboration between 57 medical institutions in the U.S. - called the National COVID-19 Convalescent Plasma Project - reported in a preprint that the remedy appears to be safe.
Last month, among its founders, Dr. Arturo Casadevall from Johns Hopkins University, told Medical News Today that near 12,000 COVID-19 patients in the U.S. had already received the procedure.
However, he was concerned that patients weren't getting the remedy early enough because it happens to be only approved for cases that are serious or life threatening.
An infusion of antibodies against the virus to improve the body’s immune response could be far better earlier in the illness.
The reason for this is that by the time a person becomes severely ill, their disease fighting capability has truly gone into overdrive, leading to inflammation of the lungs and an excessive release of immune factors, referred to as a “cytokine storm.”
Source: www.medicalnewstoday.com