US grants emergency use authorization to Regeneron Covid-19 antibodies
US drug regulators gave emergency acceptance to a Covid-19 antibody remedy over Saturday and G20 nations pushed for global usage of vaccines mainly because the pandemic resulted in further closures in elements of the world.
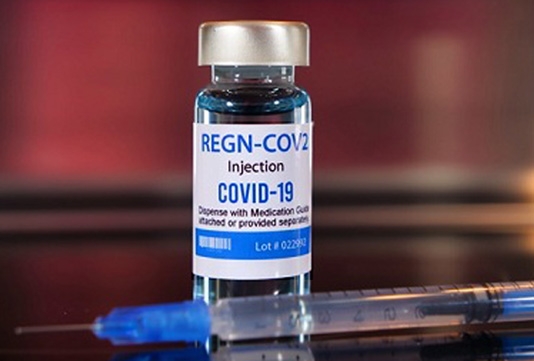
The authorization was given on Saturday to Regeneron Pharmaceuticals Inc's COVID-19 antibody remedy that Trump said helped cure him of the condition.
The green light for drugmaker Regeneron came after REGEN-COV2, a mixture of two lab-made antibodies, was proven to lessen Covid-19-related hospitalizations or er visits in patients with underlying conditions.
Regeneron's antibody treatment is the second man made antibody treatment to get an emergency use acceptance (EUA) from the FDA after an identical therapy produced by Eli Lilly was granted the position on November 9.
The human immune system naturally develops infection-fighting proteins called antibodies -- but because not everyone mounts an sufficient response, companies like Regeneron and Lilly have created lab-made solutions.
They job by binding to a good area protein of the SARS-CoV-2 virus and stopping it from invading human being cells.
The FDA said the info supporting Regeneron's EUA came from a clinical trial in 799 non-hospitalized patients with slight to average symptoms of Covid-19.
