Beximco Pharma to market global drug maker Mylan’s products
Generic drug manufacturer Beximco Pharmaceuticals yesterday penned a deal to start the sale of medication for cancer, arthritis rheumatoid and other diseases created by US-based Mylan, in what can be viewed as a stroke of good news for the country's healthcare sector.
Under the agreement, Beximco Pharma will be allowed to launch Mylan's portfolio of key monoclonal antibodies for different types of cancer, arthritis rheumatoid, Crohn's disease, ulcerative colitis and other medical conditions, said the listed company in a news release.
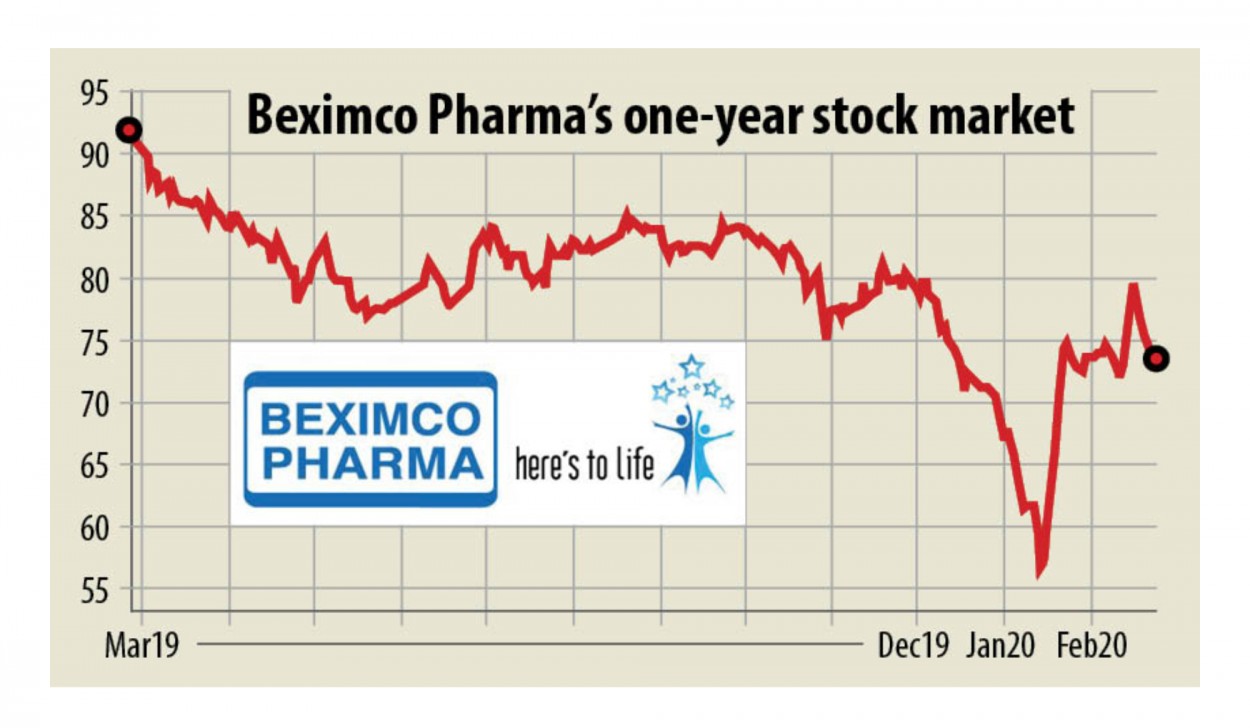
The news of this tie-up though didn't boost Beximco stock: it closed 0.41 per cent lower at Tk 73.30 yesterday.
The first product, a breast cancer drug called Ogivri, will become available to the general public in the first quarter of the existing year.
Cancer is now among the leading factors behind death in Bangladesh: a lot more than 50,000 women have tested positive for breast cancer.
Ogivri, which is approved by the united states Food and Drug Administration (FDA) and in addition received marketing authorisation from the European Medicines Agency, is biosimilar to Roche's blockbuster drug Herceptin. Its global sales in 2018 exceeded $7 billion.
The growing rate of breast cancer patients is a significant public health concern in developing markets, said Rakesh Bamzai, president of Mylan's India and emerging markets operations.
"As a worldwide leader in the development of complex pharmaceuticals, Mylan, through its commercial agreement with Beximco Pharma, is very happy to make its products accessible for patients in Bangladesh," he added.
With just about the most different portfolios for biosimilar medicines that are approved in over 80 countries, Mylan is an excellent partner for Beximco, according to Nazmul Hassan, managing director of Beximco Pharma.
At the moment, Beximco Pharma produces more than 300 generics.
With a global footprint spanning more than 50 countries, the business has been accredited by leading global regulatory authorities, which include the FDA, Malta Medicines Authority (EU), TGA (Australia), Health Canada, GCC (Gulf) and TFDA (Taiwan).
Mylan offers a growing portfolio with an increase of than 7,500 of their products being marketed across 165 countries and territories.
The US-based company also provides antiretroviral therapies, which approximately 40 % of the world's HIV/AIDS afflicted population depend on.
